BOC Sciences has developed various types of efficient and highly coordination ability chiral ligands for asymmetric reaction, and further utilizes these ligands to complete the synthesis of chiral compounds. Our chemical engineering and process research departments have dedicated organometallic chemists with extensive screening expertise. In addition, we are equipped with specialized equipment capable of efficiently producing large quantities of chiral auxiliaries and catalysts.
Enantioselective metal-catalysis is one of the most importants synthetic approaches for preparing enantioenriched compounds, which occupy a central position in areas ranging from medicinal chemistry to chiral materials. Most metal-organocatalysts are acombination of optically pure ligands and transition metal ions in various oxidation states. Among various types of chiral ligands, chiral phosphine ligands are the most frequently employed, owing to their inherent ability to strongly coordinate with transition metals and promote catalytic reactions. Mechanistically, phosphine ligands coordinate with metal centers to create a chiral pocket around the reaction centers, which eventually affects enantioselectivity and reaction rate.
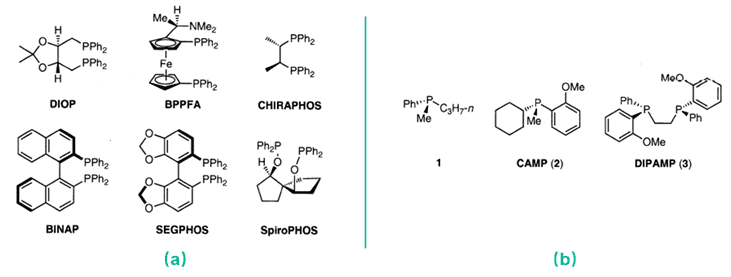 Fig. 1. Representative examples of optically active phosphine ligands (J. Synth. Org. Chem. Jpn. 2007, 65(11): 1060-1069).
Fig. 1. Representative examples of optically active phosphine ligands (J. Synth. Org. Chem. Jpn. 2007, 65(11): 1060-1069).
Chiral phosphine ligands reported so far are classified roughly into two types. One type is phosphines having asymmetric carbon atoms or backbone chirality. The stereochemical feature of this type is that the two substituents on the phosphorus atoms are diastereotopic and are capable of forming an asymmetric environment around the reaction center (Fig 1a). The other type is P-chiral phosphine ligands that possess stereogenic centers on the phosphorus atoms themselves. These ligands played very important roles in the history of homogeneous asymmetric hydrogenation (Fig 1b). Compared with the traditional chiral phosphine ligand based on carbon center chirality, chiral phosphine ligands with phosphine-centered chirality can establish a chiral environment closer to the metal center, so it may have better stereoinduction performance than the phosphine ligand with carbon center chirality.
Studies have shown that asymmetric organocatalysis is an effective way to synthesize chiral intermediates and APIs. The catalytic performance of chiral catalyst is determined not only by the metal center but also by the chiral ligand selected. Chiral phosphine ligands play pivotal roles in transition-metal-catalyzed asymmetric reactions due to their stable structure, simple synthesis, and ease of modification. Currently, chiral phosphine ligands are widely used for asymmetric hydrogenation, asymmetric conjugate addition, asymmetric allylation, asymmetric heck reaction, asymmetric cycloaddition reaction and so on.
References:

Please click here to learn more about chiral technical support.
Suitable for your research and production needs. BOC Sciences provides the most comprehensive products and services. 24 hours customer support!



