BOC Sciences possesses advanced laboratory equipment and unique R&D expertise to support the development and manufacture of multiple chiral compounds ranging from chiral catalysts, chiral ligands, chiral auxiliaries to chiral resolution reagents. We strictly monitor our products during development and research processes to ensure our customers receive first-class services and products.
Cinchona alkaloids, which were once known for the popular antimalarial drug quinine, have emerged as the most powerful class of compounds in the realm of asymmetric organocatalysis during the last 2 decades. Apart from natural cinchona alkaloids, many derivatives, such as those containing hydroxyl groups, amines, ureas, and thiourea functionalities, especially at the C9 position, either alone or in the presence of an additional catalyst that might be a simple achiral compound or metal salt, have been employed in diverse types of enantioselective syntheses by asymmetric catalysis. This can be attributed to the abundance of cinchona alkaloids in nature, their commercial availability at reasonable prices, stability and easy handling in laboratory, and their convenient modification by simple reactions.
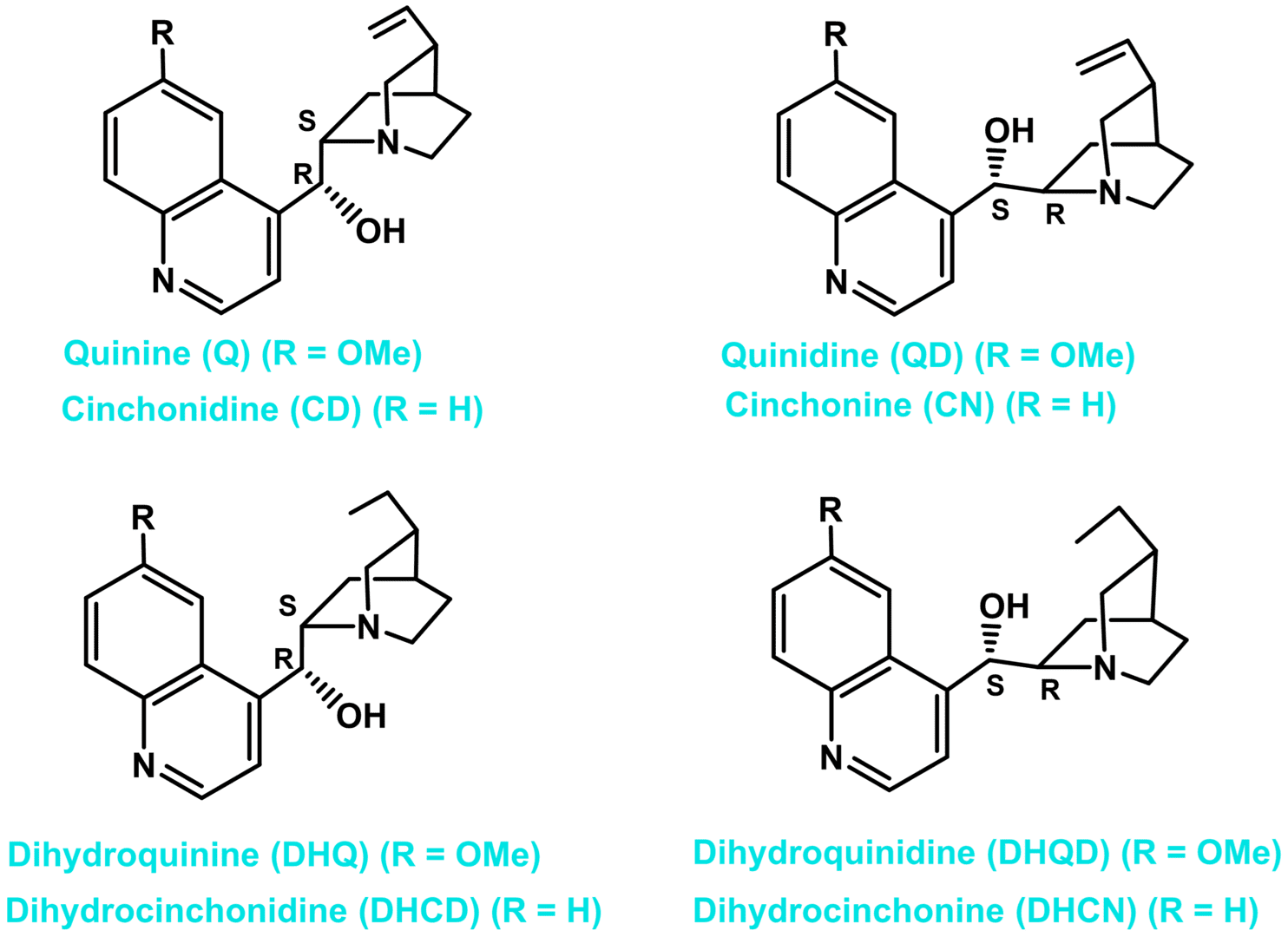 Fig.1. Structures of eight major cinchona alkaloids.
Fig.1. Structures of eight major cinchona alkaloids.
The cinchona skeleton consists of two rigid rings: an aliphatic quinuclidine and an aromatic quinoline ring joined together by two carbon-carbon single bonds. There are five stereocenters in the molecule. The cinchona alkaloids occur in pairs, which differ in configurations at C8, C9, and N1 positions. The eight major cinchona alkaloids (Fig. 1) are diastereomers, usually referred to as pseudoenantiomers because they offer enantiomeric products when used as catalysts. Several studies have shown that cinchona compounds work as bifunctional catalysts. The quinuclidine ring, bearing a tertiary nitrogen atom, is 103 times more basic in comparison to quinoline nitrogen. The tertiary nitrogen is thus responsible for the basic character of the catalyst. The H-bonding groups, such as the hydroxyl group, urea, and thiourea at the C9 position, activate the electrophile by hydrogen bonding. The relative orientation of the two rings quinoline and quinuclidine creates a “chiral pocket” around the reactive site, forcing a particular approach of the substrates, resulting in enantioselective product formation.
Since the pioneering works of H. Wynberg in the late 1970s and early 1980s, cinchona alkaloids have been intensively applied as either standalone catalysts or chiral ligands in catalytic asymmetric reactions and are now regarded as one of the most privileged chirality inducers. Indeed, today, nearly all classes of organic reactions can be effectively carried out with the use of cinchona alkaloids in a highly stereoselective fashion. Some of them are even used in large-scale processes, for example, the heterogeneous hydrogenation of α-ketoesters catalyzed by cinchona alkaloid-modified platinum, the Sharpless asymmetric dihydroxylation of olefins, and the asymmetric alkylation of indanones using cinchona alkaloid-derived chiral phase-transfer catalysts, and so on. Of these reactions, the osmium-catalyzed asymmetric dihydroxylation of olefins using cinchona alkaloid derivatives as chiral ligands has had the greatest impact on modern asymmetric catalysis.
References

Please click here to learn more about chiral technical support.
Suitable for your research and production needs. BOC Sciences provides the most comprehensive products and services. 24 hours customer support!



