Oxazolidinones are a class of chiral auxiliaries widely used in the asymmetric reaction to synthesize biologically active compounds. As a professional technical service company in the field of chiral compounds, BOC Sciences offers multiple chiral oxazolidinone auxiliaries for stereoselective protection in asymmetric synthesis.
Utilization of chiral auxiliaries is one of the main strategies for the synthesis of enantiopure compounds. Currently, a series of efficient chiral auxiliaries are commonly used in carbon-carbon bond formation with high stereoselectivity and in the synthesis of natural compounds and pharmacologically active compounds. Among them, chiral oxazolidinones developed and introduced, by David Evans in 1981, have proven to be the remarkable standard, in comparison with all others, introduced, previously. After their discovery and introductions, Evans’ oxazolidinones have been successfully employed as ideal chiral auxiliaries to control face-selectivity in enolate reactions.
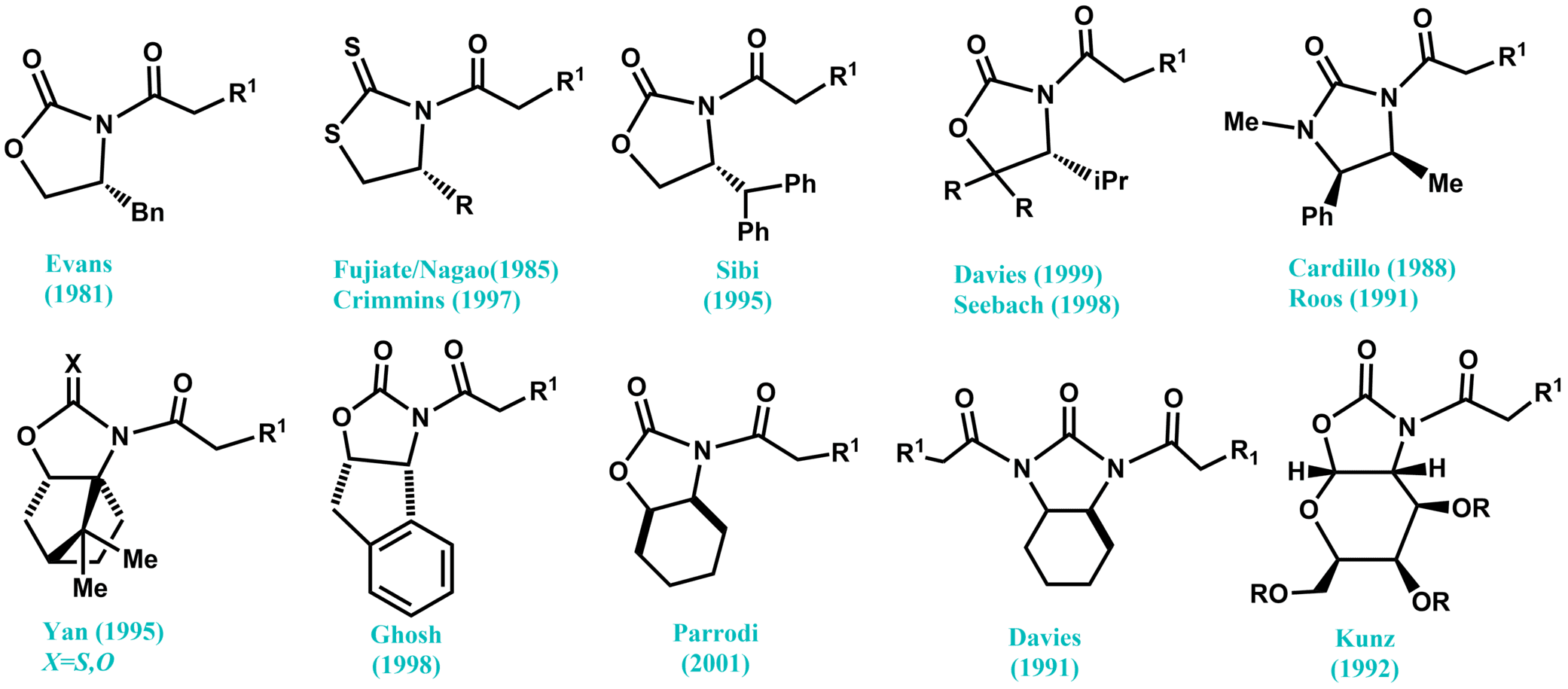 Fig. 1. Selected modified derivatives of chiral oxazolidinone.
Fig. 1. Selected modified derivatives of chiral oxazolidinone.
As the most prevalent chiral auxiliaries, Evans' 2-oxazolidinones derived from the appropriate α-amino acid, can be effectively utilized for the stereoselective formation of C–C and C–X (X = O, N, Br, F, etc.) bonds. Due to their efficiency in asymmetric formation of C–C bonds, Evans' oxazolidinone systems have been extensively studied from different points of view. Either enantiomeric forms of a broad range of Evans’ oxazolidinones are easily accessible in either form natural abundant and commercially available amino acids, such as valinole (related amino alcohols), or from ephedrine provided from natural sources. In addition to the original oxazolidinones introduced by Evans, several other modified derivatives have also been developed over the years and exploited in asymmetric synthesis. It is worth noting that some oxazolidinones deliver better selectivity in some specific reactions, inducing a high diastereoselectivity of products after crystallization.
Evans' chiral auxiliaries represent one of the most widely used auxiliaries in asymmetric total synthesis. The most prominent applications of oxazolidinones undoubtedly occur in α-alkylation, syn-aldol, 1,4-addition, and intramolecular Diels-Alder cycloaddition reactions. Evans' chiral auxiliaries have also been applied in anti-aldol reactions, Michael additions, additions to C=O and C=N bonds, and intramolecular and intermolecular cycloadditions, among others. Among them, asymmetric aldol reactions are one of the most important reaction. The first examples of the asymmetric aldol reaction, relied on utilization of a chiral pool resulted in the synthesis of optically pure β-hydroxy carbonyl compounds. In this strategy, an aldol donor attached to a chiral auxiliary was subjected to diastereoselective addition to an aldol acceptor to provide an enantio-pure intermediate. Thus, the carbonyl donors attached to optically pure oxazolidinones and their analogs, which are easily obtainable from α-amino acids, are frequently used, as dependable chiral auxiliaries for asymmetric aldol reactions.
References

Please click here to learn more about chiral technical support.
Suitable for your research and production needs. BOC Sciences provides the most comprehensive products and services. 24 hours customer support!



