| Catalog No. |
Name |
CAS |
Inquiry |
| BCC-00156 |
(R)-N-(2,6-Bis(4-(tert-butyl)phenyl)-4-oxido-7a,8,9,10,11,11a,12,13,14,15-decahydrodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
1261302-66-2 |
|
| BCC-00157 |
(S)-N-(2,6-Bis(4-(tert-butyl)phenyl)-4-oxido-7a,8,9,10,11,11a,12,13,14,15-decahydrodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
1643958-21-7 |
|
| BCC-00158 |
(S)-1,1,1-Trifluoro-N-(4-oxido-7a,8,9,10,11,11a,12,13,14,15-decahydrodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)methanesulfonamide |
2304917-54-0 |
|
| BCC-00159 |
(R)-N-(2,6-Bis(3,5-dimethylphenyl)-4-oxido-7a,8,9,10,11,11a,12,13,14,15-decahydrodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
|
|
| BCC-00160 |
(S)-N-(2,6-Di(naphthalen-1-yl)-4-oxido-7a,8,9,10,11,11a,12,13,14,15-decahydrodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
|
|
| BCC-00162 |
1,1,1-Trifluoro-N-[(11aR)-10,11,12,13-tetrahydro-5-oxido-3,7-bis(triphenylsilyl)diindeno[7,1-de:1',7'-fg][1,3,2]dioxaphosphocin-5-yl]methanesulfonamide |
|
|
| BCC-00163 |
1,1,1-Trifluoro-N-[(6R)-6-oxido-4,8-bis[4-(tert-butyl)phenyl]dibenzo[d,f][1,3,2]dioxaphosphepin-6-yl]methanesulfonamide |
|
|
| BCC-00164 |
1,1,1-Trifluoro-N-[(6S)-6-oxido-4,8-bis(3,5-dimethylphenyl)dibenzo[d,f][1,3,2]dioxaphosphepin-6-yl]methanesulfonamide |
|
|
| BCC-00165 |
1,1,1-Trifluoro-N-[(6S)-6-oxido-4,8-bis[4-(tert-butyl)phenyl]dibenzo[d,f][1,3,2]dioxaphosphepin-6-yl]methanesulfonamide |
|
|
| BCC-00166 |
1,1,1-Trifluoro-N-(3aS,8aS)-4,4,8,8-tetrakis(3,5-di-tert-butylphenyl)-2,2-bis(4-fluorophenyl)-6-hydroxy-tetrahydro-6-oxide-[1,3]dioxolo[4,5-e][1,3,2]dioxaphosphepine-6-yl)methanesulfonamide |
|
|
| BCC-00492 |
(S)-N-(2,6-Di([1,1'-biphenyl]-4-yl)-4-oxido-7a,8,9,10,11,11a,12,13,14,15-decahydrodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
|
|
| BCC-00493 |
(11bS)-N-(2,6-Bis(4-(tert-butyl)phenyl)-4-oxidodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
2417213-21-7 |
|
| BCC-00494 |
N-((11bR)-Dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide triethylamine salt |
1150592-92-9 |
|
| BCC-00495 |
N-((11bS)-2,6-Di([1,1':3',1''-terphenyl]-5'-yl)-4-oxidodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
2829289-15-6 |
|
| BCC-00496 |
(11BS)-N-(2,6-bis(4-nitrophenyl)-4-oxidodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
1628940-60-2 |
|
| BCC-00497 |
(11bR)-N-(2,6-Bis(3,5-dimethylphenyl)-4-oxido-8,9,10,11,12,13,14,15-octahydrodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
2757287-65-1 |
|
| BCC-00498 |
(11bS)-N-(2,6-Di([1,1'-biphenyl]-4-yl)-4-oxidodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
2222149-77-9 |
|
| BCC-00499 |
N-((11bS)-2,6-Dimethyl-4-oxidodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
2757287-62-8 |
|
| BCC-00501 |
(11bR)-N-(2,6-Bis(4-trifluoromethylphenyl)-4-oxidodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
2757287-36-6 |
|
| BCC-00502 |
(11bR)-N-(2,6-bis(4-nitrophenyl)-4-oxido-8,9,10,11,12,13,14,15-octahydrodinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-1,1,1-trifluoromethanesulfonamide |
2758008-05-6 |
|
For Research Use Only.
BOC Sciences possesses advanced laboratory equipment and unique R&D expertise to support the development and manufacture of multiple chiral compounds ranging from chiral catalysts, chiral ligands, chiral auxiliaries to chiral resolution reagents. We strictly monitor our products during development and research processes to ensure our customers receive first-class services and products.
Introduction
Chiral phosphoric acid diesters derived from 1,1'-bi-2-naphthol (binol) and their analogues are a highly efficient class of metal-free Brønsted acid organocatalysts. Transfer hydrogenation as well as various addition reactions to aldimines and ketimines are among the most important reactions that can be carried out efficiently with these catalysts. The moderate acidity of these catalysts renders them ideal for the activation of basic imines by hydrogen bonding or ion pairing in a dual or bifunctional fashion. However, the activation of less basic carbonyl functionalities is more demanding, and only few enantioselective applications have been reported so far. Therefore, the more acidic chiral binol-derived N-triflyl phosphoramides (NTPAs) were introduced and proved suitable for the activation of more challenging substrates. Asymmetric C-C and C-X bond forming transformations, such as Diels-Alder and [3+2] cycloaddition reactions, the Nazarov cyclization, asymmetric protonation, and Mukaiyama aldol reactions, have been carried out efficiently with chiral N-triflyl phosphoramides in a variety of organic solvents, including halogenated as well as aromatic solvents. Furthermore, Brønsted acid organocatalysts based on bis-(sulfonyl)imides were introduced by Berkessel et al. (JINGLE).
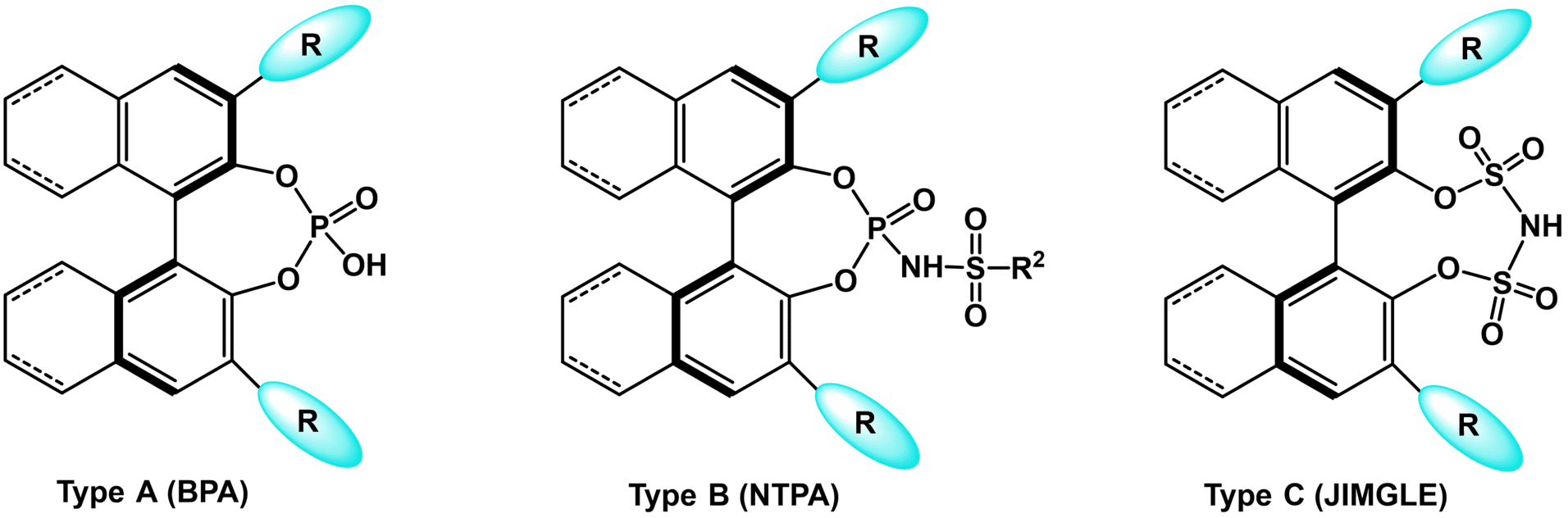 Fig. 1. Types of chiral Brønsted acid organocatalysts.
Fig. 1. Types of chiral Brønsted acid organocatalysts.
Chiral N-triflyl phosphoramides were easily synthesized from optically active BINOL derivatives by phosphorylation with POCl3 and amidation between resultant phosphoryl chloride and TfNH2. The nature of the acidity center had the strongest influence on the acidity of the catalysts studied. On the basis of reported data for achiral Brønsted acids, the acidity of these classes of compounds increases in the order: binol-derived phosphoric acid diesters (BPAs) < NTPAs < JINGLEs. The acids can be divided according to their acidity centers into three groups: A) binol-derived phosphoric acid diesters (BPAs), with pKa values in the range of 12-14, B) mixed imides of phosphoric and triflic acid (NTPAs), with pKa values in the range of 6-7, and C) imides of sulfonic acids (JINGLEs), with pKa values around 5.
Application
Chiral Brønsted acid-mediated organic reactions have proved to be synthetically useful due to their broad applicability, high enantioselectivity, and environmentally friendly conditions. Among several different types of Brønsted acid catalysts, binol-derived phosphoric acid and N-triflyl phosphoramide catalysts have been actively studied in various enantioselective reactions such as the Mannich reaction, Diels-Alder reaction, alkylation of α-diazoetser, aza-ene-type reaction, Friedel-Crafts alkylation, carbonyl-ene reaction, and hydrogenation. Among them, the reaction catalyzed by chiral N-triflyl phosphoramide displayed broader substrate scope.
References
- Leito, I. et al. On the Acidity and Reactivity of Highly Effective Chiral Brønsted Acid Catalysts: Establishment of an Acidity Scale. Angew. Chem. Int. Ed. 2013, 52, 1-5.
- Berkessel, A. et al. Synthesis and Structural Characterization of a New Class of Strong Chiral Brønsted Acids: 1,1′-Binaphthyl-2,2′-bis(sulfuryl)imides (JINGLEs). Eur. J. Org. Chem. 2010, 5165.
Related Sections:
Chiral Technical Information
 Fig. 1. Types of chiral Brønsted acid organocatalysts.
Fig. 1. Types of chiral Brønsted acid organocatalysts.



