BOC Sciences possesses advanced laboratory equipment and unique R&D expertise to support the development and manufacture of multiple chiral compounds ranging from chiral catalysts, chiral ligands, chiral auxiliaries to chiral resolution reagents. We strictly monitor our products during development and research processes to ensure our customers receive first-class services and products.
The synthesis of optically pure compounds is increasingly in demand in the pharmaceutical, fine chemicals, and agriculture industries. Asymmetric organocatalysis is one of the important strategies for the preparation of optically pure compounds with high yield and enantioselectivity, which has made significant advances in the last decades. Until now, various activation strategies have been developed, such as noncovalent catalysis via hydrogen-bonding, Brønsted base, Brønsted acid, phase transfer, and covalent catalysis via Lewis base. Chiral squaramides organocatalysts have been intensively investigated within the area of molecular recognition because of their strong hydrogen-bonding activity. It pioneered by Rawal’s group and later developed by other groups with incorporating the squaramide moiety as a powerful hydrogen bond donor in different chiral scaffolds, have been identified as another complementary or even superior H-bond donor catalyst in asymmetric organocatalysis in the past several years.
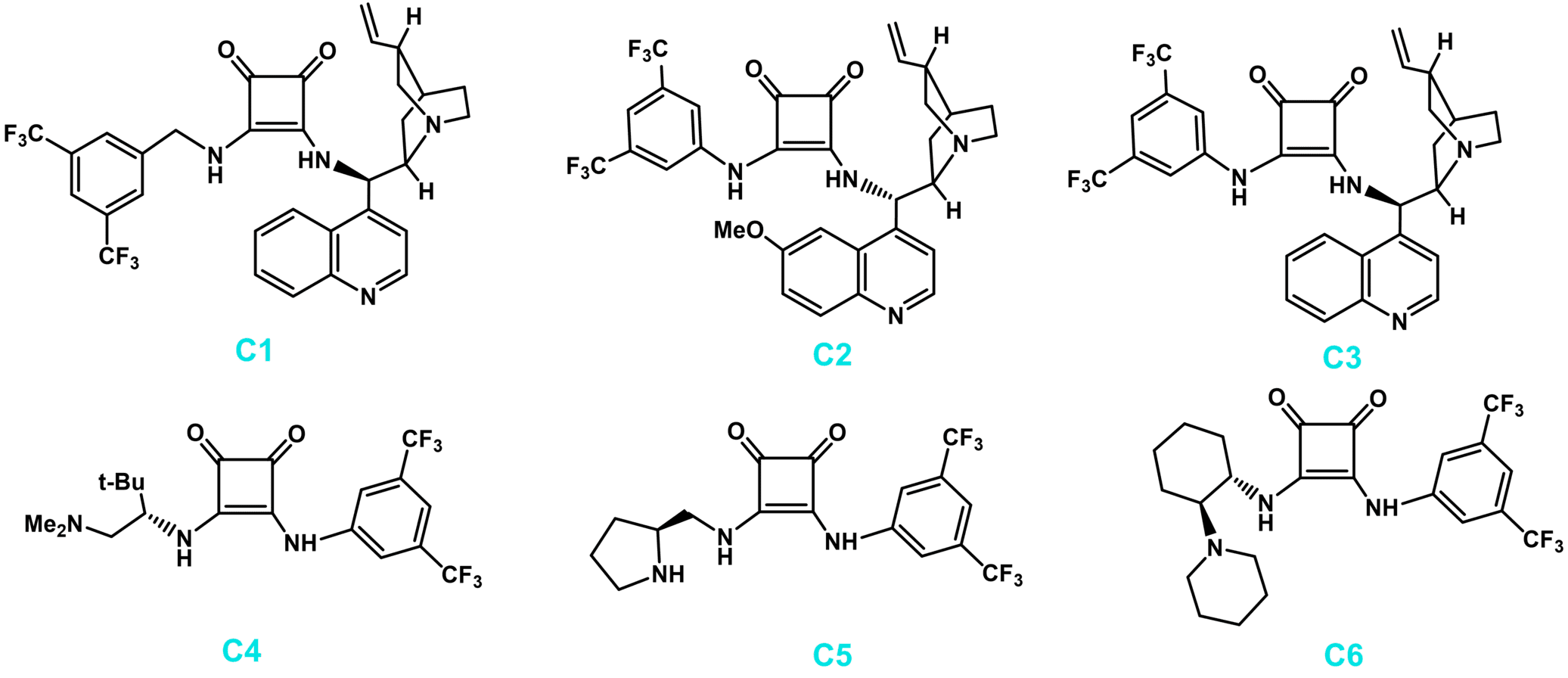 Fig. 1. Representative chiral squaramide organocatalysts (Chem. Rec. 2016, 16, 897-906).
Fig. 1. Representative chiral squaramide organocatalysts (Chem. Rec. 2016, 16, 897-906).
Squaramide’s dual binding ability, structural rigidity, further apart H-bond spacing and lower pKa values of the N-H protons, may lead to a broader substrate scope, improved recognitions, better stereoinduction or rate enhancement compared to the thiourea or urea analogues in some cases. The first chiral bifunctional squaramide derived from cinchonine was prepared a decade ago, and successfully used in the enantioselective nitro-Michael reaction. Since then, a lot of squaramides, specially derived from cinchone alkaloids or trans 1,2-cyclohexane diamine, have been synthesized and used as organocatalysts in different enantioselective transformations. Additionally, a few squaramides with a common 3,5-bis(trifluoromethyl) aniline and a chiral diamine derived from α-amino acids have been described as excellent catalysts for different Michael additions, and tandem reactions leading to complex structures.
As the rigidity of the squaramide system can provide well defined chiral pockets, the easy substitution of the squaric acid enabled the synthesis of a variety of chiral squaramide organocatalysts. Squaramides have found a variety of applications, and comprehensive reviews of the electronic and structural properties of squaramides have been published. In 2008, its excellent performance in Michael addition reaction was disclosed by Rawal and coworkers. Inspired by this realization, squaramide-derived organocatalysts were applied in various asymmetric transformations. Series of optically pure compounds, such as indoles, tetrahy droxanthones, chromenes, quinazoline, naphthoquinones, and substituted amino acid derivatives, which are widely distributed in pharmaceutical compounds and complex natural products, were synthesized in recent years.
References

Please click here to learn more about chiral technical support.
Suitable for your research and production needs. BOC Sciences provides the most comprehensive products and services. 24 hours customer support!



