Chiral auxiliaries are compounds that induce product stereoselectivity in asymmetric synthetic reactions. Multiple stereocompounds with single configuration can be obtained when using chiral auxiliaries. As a professional technical service company in the field of chiral compounds, BOC Sciences offers multiple chiral auxiliaries for stereoselective induction in asymmetric synthesis.
Asymmetric reactions using chiral auxiliaries have made significant progress over the past decades and continue to this day. Chiral auxiliaries are important tools for constructing highly complex chiral molecules. The wide availability of starting materials, the easy and versatile removal of auxiliary groups, and the applicability of chiral molecules in a variety of stereoselective transformations make them valuable intermediates and building blocks in asymmetric synthesis.
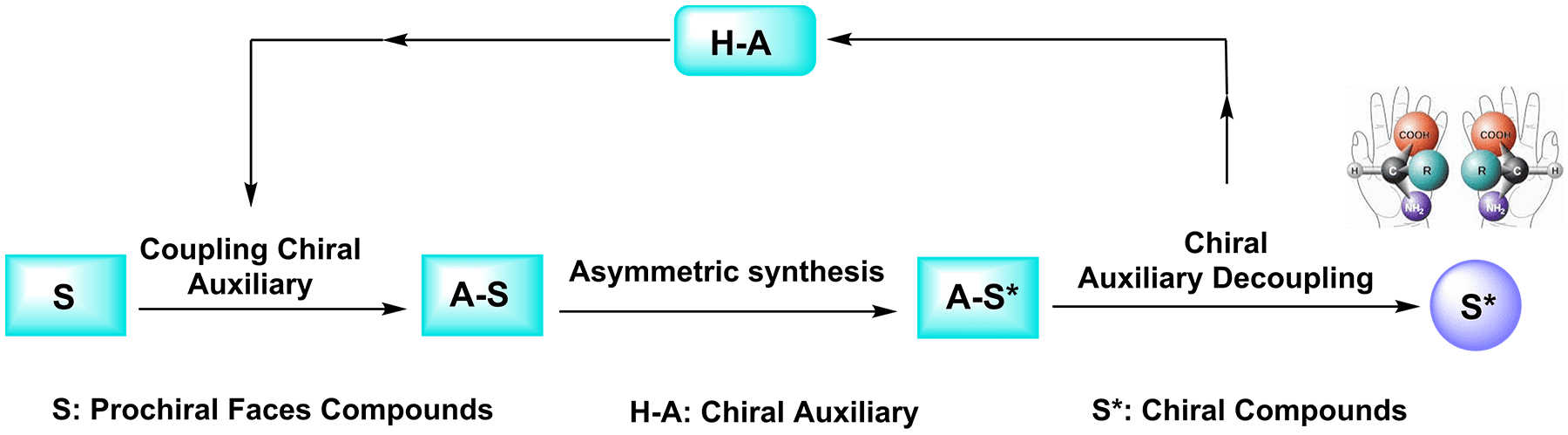
A series of efficient chiral auxiliaries are commonly used for the formation of carbon-carbon bonds with high stereoselectivity and the synthesis of natural compounds with significant pharmacological activities. In addition to the commonly used Evans' chiral auxiliaries, several chiral auxiliaries are widely used in asymmetric organic synthesis reactions: (a) Chiral amino acids are widely used as chiral auxiliaries because they can be obtained from a large number of natural sources or synthesized at low cost and their reaction products have high stereoselectivity. (b) Corey introduced (−)-8-phenylmenthol in 1975 when developing a method for obtaining a chiral intermediate for the synthesis of prostaglandins (PGs). This auxiliary induces diastereoselectivity through π-stacking interactions. Along with its enantiomer, (+)-8-phenylmenthol , Corey's auxiliary is a useful tool in chiral resolution; differentiation of the prochiral faces; and stereochemical control in cycloaddition reactions, 1,2- and 1,4-additions, oxidations, reductions, photochemical reactions, and radical reactions. (c) (S)-1-Amino-2-methoxymethylpyrrolidine (SAMP) was introduced by Enders and Eichenauer in 1976 as a chiral auxiliary for the formation of hydrazones. (d) Oppolzer's auxiliary, also known as bornanesultam or camphorsultam, was developed by Wolfgang Oppolzer and named in honor of the long-serving camphor molecule. This auxiliary is commercially available as (1R)-(+)-2,10-camphorsultam and its (1S)-(−)-enantiomer. Both molecules are versatile chirality inducers and find application in Michael reactions, Claisen rearrangements, and cycloaddition reactions, among others. (e) Carbohydrates are naturally occurring compounds of low economic value that can be used as chiral auxiliaries to provide stereoselective and regioselective products because of their various functional groups and defined stereocenters. These properties make them useful tools in the asymmetric synthesis of natural products.
Asymmetric synthesis plays a key role in the chemical production of therapeutic and natural product compounds in certain enantiomeric forms. Many pure enantiomerically compounds can be efficiently synthesized by using chiral auxiliaries to control the stereochemical outcomes of various synthetic routes. Chiral auxiliaries derived from naturally occurring compounds, such as amino acids, carbohydrates, and terpenes, are considered essential tools for the construction of highly complex molecules. By temporarily attaching these structures to organic compounds, chiral auxiliaries can influence the stereoselectivity of reactions. Once the stereoselective protection is no longer required, the chiral auxiliary can be removed and recycled.
References

Please click here to learn more about chiral technical support.
Suitable for your research and production needs. BOC Sciences provides the most comprehensive products and services. 24 hours customer support!



