BOC Sciences possesses advanced laboratory equipment and unique R&D expertise to support the development and manufacture of multiple chiral compounds ranging from chiral catalysts, chiral ligands, chiral auxiliaries to chiral resolution reagents. We strictly monitor our products during development and research processes to ensure our customers receive first-class services and products.
Amino acids play a significant role in nature and in our regular life by performing a variety of functions such as catalysis (enzymes), signal transductions (neurotransmitters), defense mechanisms (antibodies), and also as drugs against many diseases. This wide palette of functions is possibly due to the ease with which eclectic structural and functional modification in the peptides that can be achieved by linking various amino acids with varied functional groups in their side chains. Proline, having the advantages of amino acids such as nontoxic, feasible, and economic, is one of the organocatalysts used for sustainable catalysis. The limited solubility of proline also eases its recovery and separation from the substrates. Proline and its derivatives are often used as asymmetric catalysts in organic reactions such as Corey-Bakshi-Shibata (CBS) reductions, proline catalyzed aldol reactions, Mannich reaction, and so on.
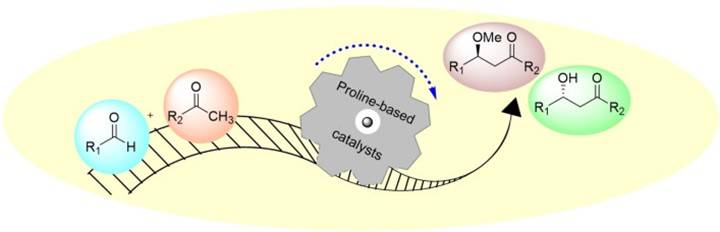 Fig. 1. Catalytic schematic of proline catalyst (European Journal of Organic Chemistry. 2021, 37: 5288-5311).
Fig. 1. Catalytic schematic of proline catalyst (European Journal of Organic Chemistry. 2021, 37: 5288-5311).
Proline is able to promote a very broad diversity of transformations, which can be explained by the multiple catalytic roles allowed by its structural features. Although a single proline can bring about addition reaction with moderate yields and stereoselectivities, its modification by attaching hydrogen bond-inducing and steric elements could increase the catalytic efficiency. Proline can behave both as Brønsted acid or base, or show both behaviors in the course of a mechanism, being therefore a bifunctional catalyst. In comparison to other amino acids, it raises the nitrogen pKa and increases its nucleophilicity. For this reason, the proline nitrogen behaves as a good nucleophile towards carbonyl groups, generating the iminium or enamine intermediates that are characteristic of covalent organocatalysis. Furthermore, the presence of the carboxylic function allows the formation of organized transition states stabilized by several hydrogen bonds, often leading to high stereoselectivities. Finally, the presence of adjacent amino and carboxylic functions leads to chelating properties, and therefore permits the participation of proline in metal-catalyzed reactions.
Since the discovery of successful proline catalyzed aldol reaction, great strides have been made in understanding and exploring the newer routes and strategies for various asymmetric reactions catalyzed by L-proline or its derivatives. Proline is now regarded as an efficient and important organocatalyst in several asymmetric transformations, such as aldol reactions, crossed aldol reactions involving different aldehydes as donors or acceptors, Mannich reactions involving ketones, aldehydes, and amines, Michael additions involving ketones and aldehydes, additions to imines, nitro alkenes, in addition to many other reactions as well. The reactivity and enantioselectivity of proline catalyzed reactions amount to a series of interactions involving proline that are comparable to enzyme catalyzed reactions, such as substrate recognition, transition state stabilization, and the resulting formation of the product. Furthermore, L-proline is an osmoprotectant and is therefore frequently used in many pharmacological as well as biotechnological applications.
References:

Please click here to learn more about chiral technical support.
Suitable for your research and production needs. BOC Sciences provides the most comprehensive products and services. 24 hours customer support!



