With advanced laboratory equipment and experienced R&D team, BOC Sciences is a leading manufacturer and supplier of various chiral resolution reagents and other chiral compounds, supplying needs for the asymmetric synthesis and any other related application.
Chiral resolution, also known as optical resolution, is used to separate racemic compounds into different mirror isomers and is an important tool for the production of optically active drugs. The resolution mechanism is that the undesired enantiomer is continuously converted to the desired enantiomer by racemization while resolving. Currently, three methods mainly be used for obtaining chiral compounds: chiral synthesis, chiral catalysis, and chiral resolution. Among them, chiral resolution is economical, simple operation, and easy for industrial production.
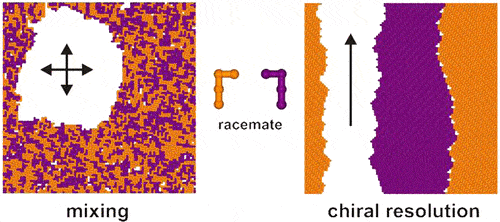 Fig. 1. Schematic diagram of chiral resolution (Langmuir. 2012, 28, 30, 11095-11105).
Fig. 1. Schematic diagram of chiral resolution (Langmuir. 2012, 28, 30, 11095-11105).
Chiral resolution reagent is a chiral auxiliary used to transform enantiomers mixtures into non-enantiomers to analyze the content of each enantiomer in the mixture. Analysis can be performed by spectroscopy or chromatography. The use and design of chiral resolution reagents should abide by the following rules to effectively determine the stereochemistry of the analyte: (a) Chiral resolution reagent must be enantiomerically pure, or the enantiomeric purity must be known accurately. (b) The reaction of the chiral resolution reagent with the two enantiomers should be accomplished under the reaction conditions. This serves to avoid enrichment or depletion of one enantiomer of the analyte by kinetic resolution. (c) Chiral resolution reagents must not be racemic under derivatization or analytical conditions. (d) The connection of chiral resolution reagent should be mild enough to ensure the substrate does not racemize. (e) When analyzed by NMR, the chiral resolution reagent should have the group that generates a singlet state NMR spectrum and the single peak must be away from other peaks.
Chromatography can be applied to separate products after the chiral resolution reagent reacted with the target analyte. The ability of chiral resolution reagents to separate chiral molecules depends on two main chromatographic mechanisms, including the differential solvation in mobile phase and the differential adsorption on stationary phase. Usually, chromatography can separate chiral compounds to bypass difficult crystallization or collect all enantiomeric in solutions. Chromatography has multiple variants (e.g. HPLC, gas chromatography, and fast chromatography) that are widely applicable to different molecules. The principles of the chiral resolution by high-performance liquid chromatography (HPLC) are divided into two categories: one is direct resolution using a chiral stationary phase (CSP), and the other relies on diastereomer formation with a suitable chiral derivatization reagent. A number of enantioseparations have been achieved with the direct methods that employ CSP columns containing immobilized chiral selectors. The separation is due to the stability difference of the diastereomeric complexes formed between the stationary phase and each enantiomer in the chromatographic system.
References

Please click here to learn more about chiral technical support.
Suitable for your research and production needs. BOC Sciences provides the most comprehensive products and services. 24 hours customer support!



