BOC Sciences possesses advanced laboratory equipment and unique R&D expertise to support the development and manufacture of multiple chiral compounds ranging from chiral catalysts, chiral ligands, chiral auxiliaries to chiral resolution reagents. We strictly monitor our products during development and research processes to ensure our customers receive first-class services and products.
Organocatalysis connotes the use of substoichiometric quantities of organic molecules in order to fasten the rate of a reaction. It constitutes one of the major domains in asymmetric catalysis, emphasizing greener approaches to organocatalysis. Notably, plenty of asymmetric C-C bond formation reactions are often catalyzed by complex organic as well as inorganic compounds such as transitional-metal catalysts, metal-organic frameworks, etc. The steadfast use of such metal-based catalysts in organic reactions is challenging mainly due to the complexity of their synthesis and development. Additionally, these catalysts tend to have detrimental impacts on the ecosystem. In this regard, environmentally benign catalysts can improve the synthesis of various important heterocycles and natural products and tackle the aforementioned limitations.
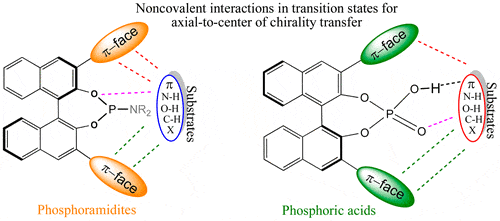 Fig. 1. Transition state models for understanding the origin of chiral induction in asymmetric catalysis (Acc. Chem. Res. 2016, 49(5): 1019-1028).
Fig. 1. Transition state models for understanding the origin of chiral induction in asymmetric catalysis (Acc. Chem. Res. 2016, 49(5): 1019-1028).
Chiral small molecule organocatalysts are non-metal small organic compounds which have carbon, nitrogen, hydrogen, sulfur and phosphorus. Because of their advantages such as non-toxicity, reduction in hazardous chemical waste, saving cost, time and energy, non-inert reaction conditions and easy to use, these pure compounds have attracted researcher’s attention for asymmetric version of various reactions. Ideally, organocatalysts must be stable and accessible through rather simple synthetic routes and on the other hand, these relatively small molecules need to be rapidly degraded after their use, without significant effluent generation. In many cases, useful starting materials are readily available from natural sources of organic molecules in enantiomerically pure form (the so-called chiral pool), including for example proteinogenic amino acids, which initially allowed the exponential development of organocatalysts.
The production of enantiopure compounds is crucial to the pharmaceutical, agrochemical, flavour, and fragrances industries, leading to a huge demand for chiral molecules. Since 2000, the study of chiral small molecule organocatalysts has become a hot field in asymmetric catalysis. Compared with chiral metal complex catalysts, the advantage of chiral small molecule organocatalysts is that most metal complex catalysts are sensitive to water and air, and need to be carried out under anhydrous and oxygen-free conditions. In addition, the chiral metal complex catalyst has high cost, high toxicity, and is not friendly to the environment, which often leads to excessive heavy metal content in the fields of medicine and food. Chiral small molecule organocatalysts can avoid these problems well and can also be recycled. Chiral secondary amines such as proline and its bifunctional derivatives, Jørgensen-Hayashi catalysts and MacMillan catalyst are among the most employed organocatalysts for a wide spectrum of substrates in several enantioselective reactions such as aldol, Michael, Mannich, Diels-Alder, α-oxidations and epoxidations, ene-reactions, among others.
References:

Please click here to learn more about chiral technical support.
Suitable for your research and production needs. BOC Sciences provides the most comprehensive products and services. 24 hours customer support!



