BOC Sciences has developed various types of efficient and highly coordination ability chiral ligands for asymmetric reaction, and further utilizes these ligands to complete the synthesis of chiral compounds. Our chemical engineering and process research departments have dedicated organometallic chemists with extensive screening expertise. In addition, we are equipped with specialized equipment capable of efficiently producing large quantities of chiral auxiliaries and catalysts.
Asymmetric catalyses with enzymes, chiral metal complexes, and chiral organic molecules have emerged as successful and powerful tools in asymmetric synthesis. Among the three catalytic asymmetric processes, artificial metal complex catalysis and organocatalysis have only a very short history compared to traditional biocatalysis but are now a predominant part of asymmetric synthesis in both research and application. Asymmetric synthesis is the main method for the construction of chiral drug and pesticide molecules, and the key to control this selectivity in metal catalysis is to find suitable chiral ligands. To this end, scientists have established many efficient chiral ligands through ingenious design in the past half century. Currently, common chiral ligands include phosphine ligands, N-heterocyclic carbene ligands, oxygen ligands, nitrogen ligands, diketone ligands, alkene ligands, etc.
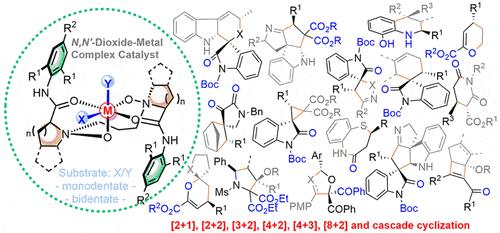 Acc. Chem. Res. 2017, 50(10): 2621-2631.
Acc. Chem. Res. 2017, 50(10): 2621-2631.
Chiral ligands are usually complicated molecules with ample possibilities of variability. The use of ligands plays a pivotal role in the activation of carbon-hydrogen and carbon-carbon bonds. Mechanistically, the coordination of ligands and metals can change their structure and reactivity, reducing the reaction activation energy. In addition, chiral ligands cooperate with substrates or catalysts to control the stereoselectivity through electronic and steric hindrance effects. Some ligands can also improve the solubility of metal catalysts in organic solvents, stabilize the catalyst, and maintain the concentration of active species in the reaction system. In addition, the catalytic efficiency is usually inconsistent when the same ligand is coordinated with different central metals or substrates, which greatly expands the adaptability of chiral ligand-modified metal catalysts.
The development of chiral compounds is of considerable interest for various fields, including pharmaceutical research, functional material development, basic chemistry, and life sciences. The large majority of chiral compounds possess their chirality at asymmetric carbon centers. However, many chiral compounds also have other elements as asymmetric centers, including sulfur, phosphorus, nitrogen, oxygen, and other heteroatoms. With the development of asymmetric catalysis, these types of ligands have proven to be indispensable due to their promising performance in numerous reactions such as adol reactions, Diels-Alder reactions, Friedel-Crafts reactions, Baylis-Hillman reactions, Mannich reactions, Michael addition reactions, etc.
References:

Please click here to learn more about chiral technical support.
Suitable for your research and production needs. BOC Sciences provides the most comprehensive products and services. 24 hours customer support!



