BOC Sciences has developed various types of efficient and highly coordination ability chiral ligands for asymmetric reaction, and further utilizes these ligands to complete the synthesis of chiral compounds. Our chemical engineering and process research departments have dedicated organometallic chemists with extensive screening expertise. In addition, we are equipped with specialized equipment capable of efficiently producing large quantities of chiral auxiliaries and catalysts.
The enantioselective syntheses of complex chiral molecules, such as those routinely demanded by the pharmaceutical industry, rank among the most important and problematic objectives in all of chemistry. Asymmetric transition metal catalysis is one of the most successful developments in modern synthetic chemistry, which simplifies the process of obtaining a variety of enantiopure chiral organic compounds. Ideally, small amounts of chiral ligands in asymmetric catalysis are sufficient to convert a large amount of achiral starting material to a more precious enantiopure chiral product. For achieving high enantioselectivity in a certain catalysed transformation, the design of the chiral ligand is crucial for its success. Among them, chiral bidentate and polydentate nitrogen-ligand metal complexes are widely used in asymmetric catalysis.
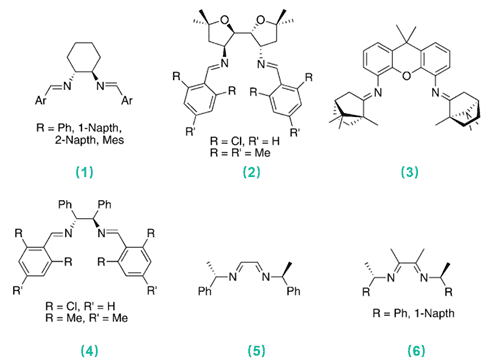 Fig. 1. Representative C2-symmetric chiral diimine ligands (Dalton Trans. 2007, 4627-4640).
Fig. 1. Representative C2-symmetric chiral diimine ligands (Dalton Trans. 2007, 4627-4640).
The development of efficient chiral ligands is a central issue in the research of asymmetric catalysis. Nitrogen-containing chiral ligands have distinct advantages, including easy preparation, high stability, recyclability, and more importantly, via the nitrogen atom that can stabilize the central metal at a high valence. For these reasons, nitrogen-containing ligands attract increasing attention and are used more and more in asymmetric catalysis. Among the large amount of nitrogen-containing ligands developed over the last decades, ligands with a C2-symmetry gave great success. For instance, chiral salen ligands showed a high efficiency in the asymmetric epoxidation of C-C double bonds, and chiral bisoxazoline and bipyridine ligands were widely used in various asymmetric catalytic processes. Currently, the chiral nitrogen ligands on the market mainly include the following categories: diimine ligand, mixed pyridyl- and pyrrolylimine ligands, oxazoline ligands, pyrrolyl- and pyridyloxazoline ligands, oxazolinylamine ligands, pyrrolidinyl oxazoline ligands, oxazolidine ligands and so on.
Chiral multidentate nitrogen-donor ligands, particularly, those bearing a pyridine ring have been widely used in transition-metal-catalyzed asymmetric catalysis because of their rich coordination chemistry. Among the reported examples, chiral tridentate pyridine-containing ligands have attracted increasing attention in recent years. Some notable examples include Pybox, IPO, BIP, BPI, and PyBidine. They have exhibited excellent catalytic performance in various asymmetric reactions, such as C-C bond forming reactions, carbonyl-ene reactions, hydrosilylation, cycloaddition, hydrogenation, and so on.
References:

Please click here to learn more about chiral technical support.
Suitable for your research and production needs. BOC Sciences provides the most comprehensive products and services. 24 hours customer support!



